AEIF Newsletter 3: Early Detection of Dementia and Depression
Early detection of diseases plays a crucial role in both Alzheimer’s Disease and depression treatment and prevention strategies. The precise time delay to a confirmed diagnosis impacts all outcomes. Expert, reliable medical evaluations (physical and cognitive) are increasingly difficult and costly to obtain in our healthcare system and rapid self assessment tools are now available. At minimum cost, we offer assessment tools to successfully detect early onset of both AD and depression.
Approximately 20% of dementia cases are unrelated to Alzheimer’s Disease and are potentially treatable. The most cost effective, reliable, and reproducible method to detect early cognitive impairment is computerized touch screen testing for mild cognitive impairment (MCI). A 2006 study was funded and published by the National Institute of Health proving the superiority of such tests, though these tests have yet to be properly instituted into our national healthcare system.
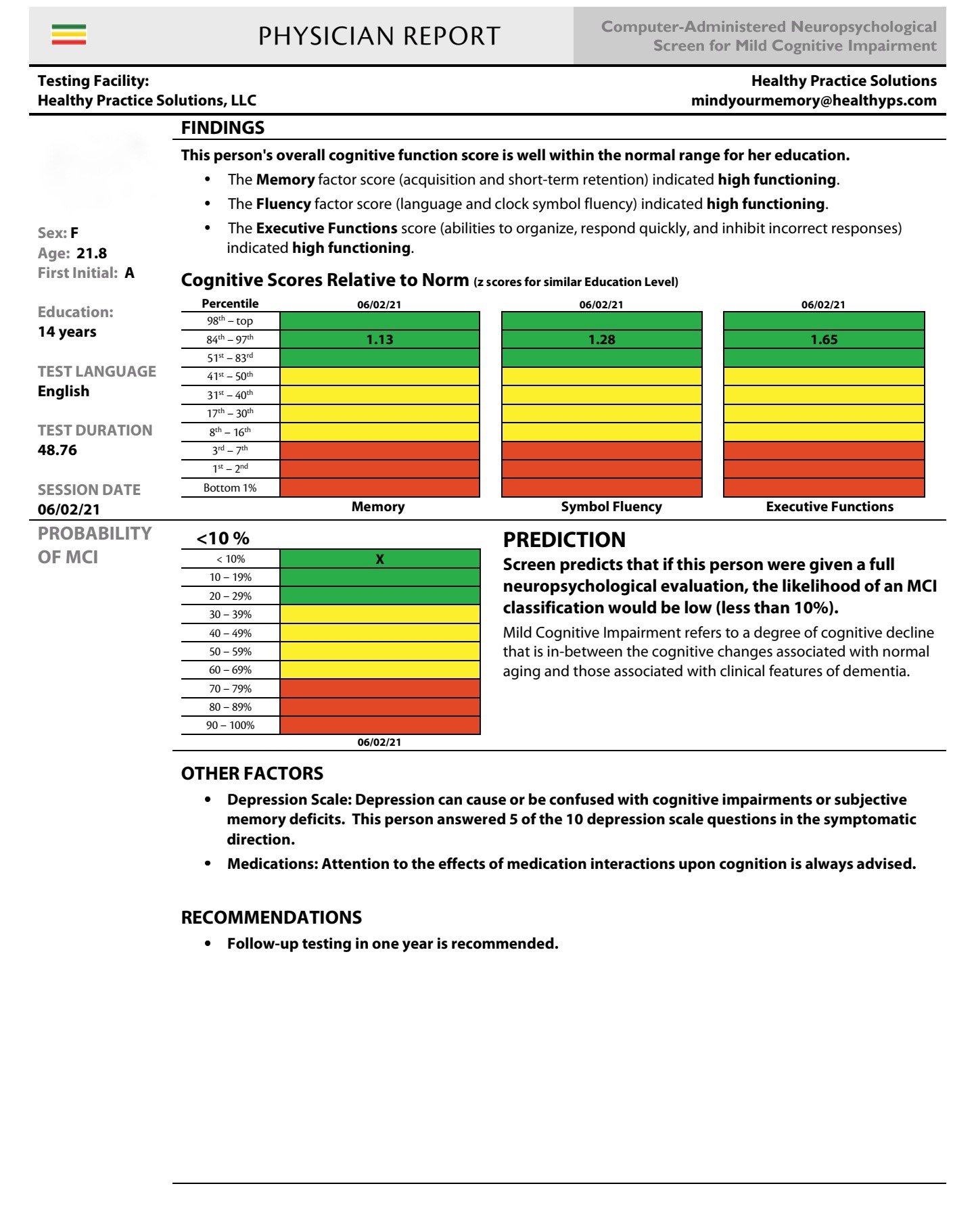
Dr. Maria Carrillo, the chief scientific officer of the Alzheimer’s Association, speaking to the Time 100 Health Summit in October, 2019, stated that people should have “cognitive assessments as a part of their routine annual healthcare”. Traditionally, an annual physical exam has never included a mental health evaluation. In so many ways, the medical profession has been remiss in not integrating a substantial mental health exam on an annual basis. Below is an individual test with normal cognition function, but indicative of significant depression previously undetected and unreported.
If you are interested in obtaining this test, please contact us at annearlyintervention.org/contact
Baseline MCI, smell and taste evaluation and continuous monitoring are imperative in the early detection process, as has been well demonstrated and documented in cases of COVID-19, depression, Parkinson’s Disease, concussion, chemo brain and other progressive neurological conditions. Individuals can track their cognitive health by taking an annual reproducible, confidential MCI exam to be compared with their baseline values. This exam detects possible changes in memory, symbolic fluency, and/or executive decision making. Any comprehensive treatment plan must continuously monitor a quantitative and reproducible MCI standard procedure in order to claim any treatment efficacy. By adopting baseline annual MCI and depression testing, the patient can have rapid deployment of new and innovative treatments as they continue to be developed. Our collaborative effort with Healthy Practice Solutions under the direction of Kevin Wolfe is integral to our goal to make these tests accessible and affordable on a confidential and repeated basis in accordance with HIPPA laws. These treatments must be monitored by this MCI computerized process for validation of any treatment or other investigational studies.